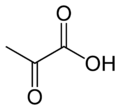
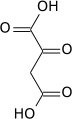
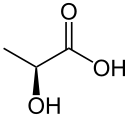
Pyruvic acid
| Pyruvic acid | |
|---|---|
 |
 |
|
2-oxopropanoic acid
|
|
|
Other names
α-ketopropionic acid; acetylformic acid; pyroracemic acid; Pyr
|
|
| Identifiers | |
| CAS number | 127-17-3 |
| ChemSpider | 1031 |
|
SMILES
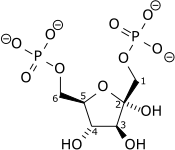
CC(C(O)=O)=O
|
|
| Properties | |
| Molecular formula | C3H4O3 |
| Molar mass | 88.06 g/mol |
| Density | 1.250 g/cm³ |
| Melting point |
11.8 °C, 285 K, 53 °F |
| Boiling point |
165 °C, 438 K, 329 °F |
| Acidity (pKa) | 2.49 at 25 °C |
| Related compounds | |
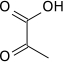
| Other anions | pyruvate ion 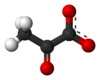 |
| Related keto-acids, carboxylic acids | acetic acid glyoxylic acid oxalic acid propionic acid acetoacetic acid |
| Related compounds | propionaldehyde glyceraldehyde methylglyoxal sodium pyruvate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
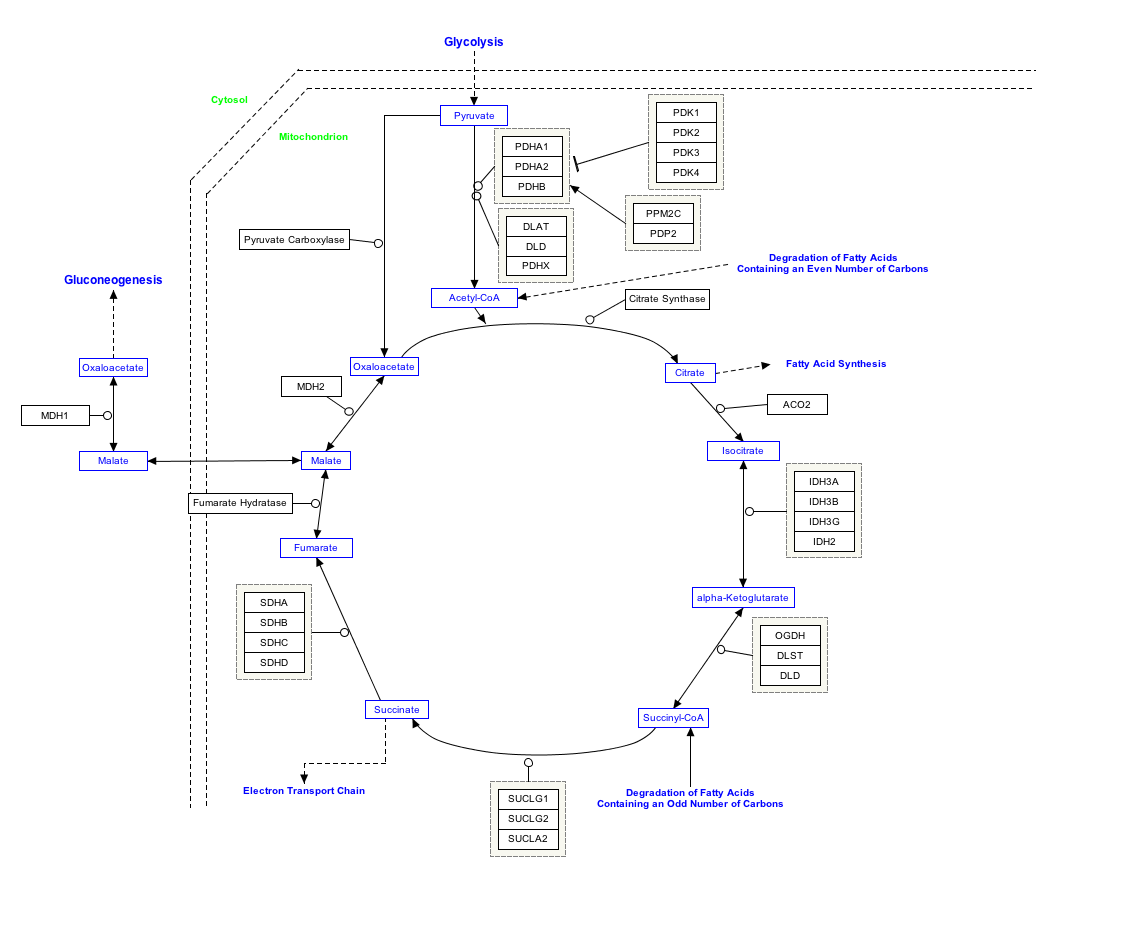
Pyruvic acid (CH3COCOOH) is an organic acid. It is also a ketone, as well as being the simplest alpha-keto acid. The carboxylate (COOH) ion (anion) of pyruvic acid, CH3COCOO-, is known as pyruvate, and is a key intersection in several metabolic pathways. It can be made from glucose through glycolysis, supplies energy to living cells in the citric acid cycle (also known as the Krebs cycle), and can also be converted to carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol.
Contents |
Chemistry
Pyruvic acid is a colorless liquid with a smell similar to that of acetic acid. It is miscible with water, and soluble in ethanol and diethyl ether. In the laboratory, pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium hydrogen sulfate, by the oxidation of propylene glycol by a strong oxidizer (eg. potassium permanganate or bleach), or by the hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:
- CH3COCl + KCN → CH3COCN
- CH3COCN → CH3COCOOH
Biochemistry
Pyruvate is an important chemical compound in biochemistry. It is the output of the anaerobic metabolism of glucose known as glycolysis. One molecule of glucose breaks down into two molecules of pyruvate, which are then used to provide further energy, in one of two ways. Pyruvate is converted into acetyl-coenzyme A, which is the main input for a series of reactions known as the Krebs cycle. Pyruvate is also converted to oxaloacetate by an anaplerotic reaction which replenishes Krebs cycle intermediates; alternatively, the oxaloacetate is used for gluconeogenesis. These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also called the citric acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.
If insufficient oxygen is available, the acid is broken down anaerobically, creating lactate in animals and ethanol in plants and microorganisms. Pyruvate from glycolysis is converted by anaerobic respiration to lactate using the enzyme lactate dehydrogenase and the coenzyme NADH in lactate fermentation, or to acetaldehyde and then to ethanol in alcoholic fermentation.
Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted into carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol. Therefore it unites several key metabolic processes.
The pyruvic acid derivative bromopyruvic acid is being studied for potential cancer treatment applications by researchers at Johns Hopkins University in ways that would support the Warburg hypothesis on the cause(s) of cancer.
Pyruvate production by glycolysis
In glycolysis, phosphoenolpyruvate (PEP) is converted to pyruvate by pyruvate kinase. This reaction is strongly exergonic and irreversible; in gluconeogenesis it takes two enzymes, pyruvate carboxylase and PEP carboxykinase to catalyze the reverse transformation of pyruvate to PEP.
| phosphoenolpyruvate | pyruvate kinase | pyruvate | |
 |
 |
||
| ADP | ATP | ||
 |
|||
| ADP | ATP | ||
| pyruvate kinase | |||
Compound C00074 at KEGG Pathway Database. Enzyme 2.7.1.40 at KEGG Pathway Database. Compound C00022 at KEGG Pathway Database.
Pyruvate decarboxylation to acetyl CoA
Pyruvate decarboxylation by the pyruvate dehydrogenase complex produces acetyl-CoA.
| pyruvate | pyruvate dehydrogenase complex | acetyl-CoA | |
 |
|||
| CoA + NAD+ | CO2 + NADH + H+ | ||
 |
|||
Pyruvate carboxylation to oxaloacetate
Carboxylation by the pyruvate carboxylase produces oxaloacetate.
| pyruvate | pyruvate carboxylase | oxaloacetate | |
 |
 |
||
| ATP + CO2 | ADP + Pi | ||
 |
|||
Transamination by the alanine aminotransferase
| pyruvate | alanine transaminase | alanine | |
 |
 |
||
| glutamate | α-ketoglutarate | ||
 |
|||
| glutamate | α-ketoglutarate | ||
Reduction to lactate
Reduction by the lactate dehydrogenase produces lactate.
| pyruvate | lactate dehydrogenase | lactate | |
 |
 |
||
| NADH | NAD+ | ||
 |
|||
| NADH | NAD+ | ||
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [1]
Origin of life
Current evolutionary theory on the origin of life posits that the first organisms were anaerobic because the atmosphere of prebiotic Earth was, in theory, almost barren of diatomic oxygen. As such, requisite biochemical materials must have preceded life. In vitro, iron sulfide at sufficient pressure and temperature catalyzes the formation of pyruvate. Thus, argues Günter Wächtershäuser, the mixing of iron-rich crust with hydrothermal vent fluid is suspected of providing the fertile basis for the formation of life.
See also
- Pyruvate scale
References
- doi:10.1126/science.289.5483.1337
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cite error: <ref> tags exist, but no <references/> tag was found